 Image credit: Unsplash
Image credit: Unsplash
Abstract
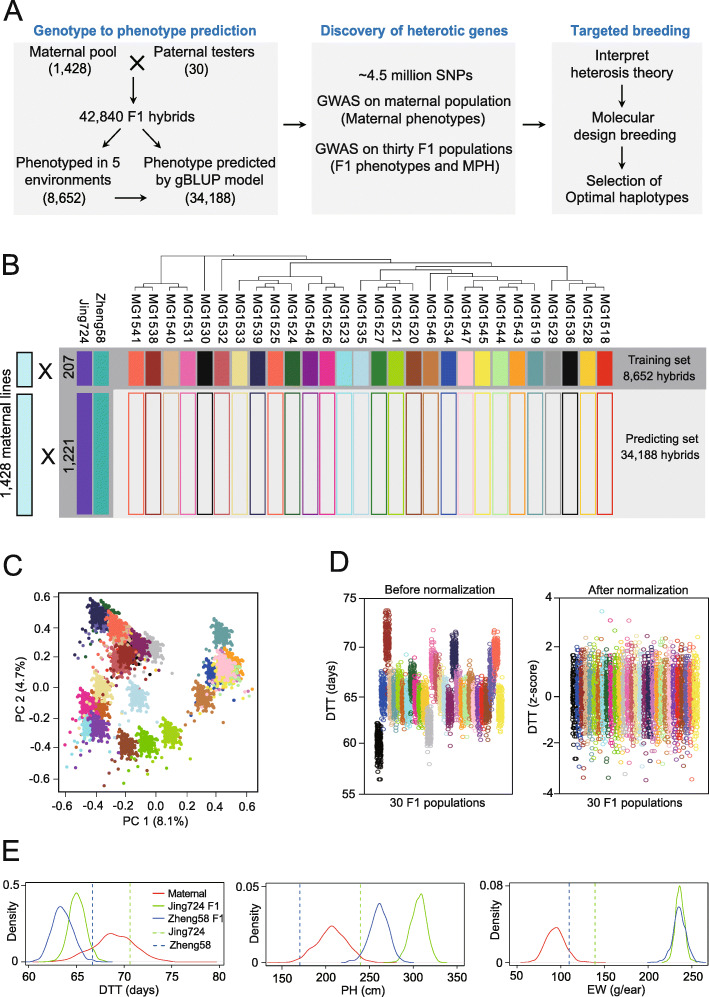
Background: In maize hybrid breeding, complementary pools of parental lines with reshuffled genetic variants are established for superior hybrid performance. To comprehensively decipher the genetics of heterosis, we present a new design of multiple linked F1 populations with 42,840 F1 maize hybrids, generated by crossing a synthetic population of 1428 maternal lines with 30 elite testers from diverse genetic backgrounds and phenotyped for agronomic traits.
Results: We show that, although yield heterosis is correlated with the widespread, minor-effect epistatic QTLs, it may be resulted from a few major-effect additive and dominant QTLs in early developmental stages. Floral transition is probably one critical stage for heterosis formation, in which epistatic QTLs are activated by paternal contributions of alleles that counteract the recessive, deleterious maternal alleles. These deleterious alleles, while rare, epistatically repress other favorable QTLs. We demonstrate this with one example, showing that Brachytic2 represses the Ubiquitin3 locus in the maternal lines; in hybrids, the paternal allele alleviates this repression, which in turn recovers the height of the plant and enhances the weight of the ear. Finally, we propose a molecular design breeding by manipulating key genes underlying the transition from vegetative-to-reproductive growth.
Conclusion: The new population design is used to dissect the genetic basis of heterosis which accelerates maize molecular design breeding by diminishing deleterious epistatic interactions.